Background
Cell division cycle 7-related protein kinase (CDC7), or DBF4-dependent CDC7 kinase (DDK), is a cell cycle kinase that maintains DNA replication by phosphorylation and activation of the minichromosome maintenance protein 2 and 4 (MCMs), components of the replicative DNA helicase. Due to the central role of CDC7 in maintenance of the replication fork integrity, chemical inhibition of CDC7 kinase can ultimately lead to cancer cell death.
SGR-2921 is an oral, small molecule inhibitor of CDC7. Preclinical studies demonstrate that, among all cell lines tested, SGR-2921 has the most potent anti-proliferative activity in AML. Potent antitumor activity has also been demonstrated in cell line-derived xenograft (CDX) and patient-derived xenograft (PDX) AML models. This antitumor response is observed in animal models representative of difficult to treat patient populations and appears to be agnostic of serious mutations (including those with p53 mutations and FLT3 mutations), BTK resistance, and multiple prior lines of treatment.
Patients with relapsed or refractory (R/R) acute myeloid leukemia (AML) or high-risk (HR) or very high-risk (VHR) myelodysplastic syndrome (MDS) have low response rates and poor overall survival. Considering the potent preclinical activity of SGR-2921 in AML and MDS models, coupled with the high unmet medical need in this population, SGR-2921 is being evaluated in patients with R/R AML and HR/VHR MDS.
Study Design and Methods
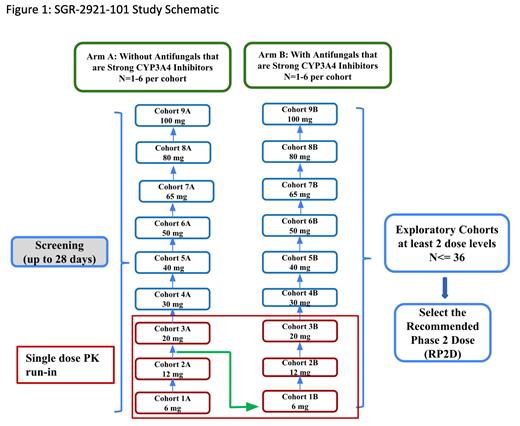
This is a phase 1, FIH, open-label, single-agent, two-arm, dose escalation study (NCT#05961839) designed to evaluate safety and tolerability and identify the recommended phase 2 dose (RP2D) of SGR-2921 as monotherapy in subjects with R/R AML, HR MDS, or VHR MDS. The study utilizes a hybrid accelerated titration design with single patient cohorts that transitions to a 3+3 design once a single Grade 2 event or DLT is observed.
This is a multicenter global study (US, Spain and France) with an estimated study recruitment start date in October, 2023. SGR-2921 will be administered orally, once daily, utilizing a 5-day on and 9-day off dosing schedule over a 28-day cycle. Up to a maximum of 144 patients will be enrolled in the dose escalation and exploratory cohort phase of the study.
To evaluate the effect of CYP3A4 inhibition on SGR-2921 exposure, subjects will be enrolled into one of two staggered, parallel study treatment arms, according to concomitant administration with (Arm B) or without (Arm A) azole antifungals that are strong CYP3A4 inhibitors at time of first dose. Safety and tolerability must be demonstrated in treatment Arm A, at the first two dose levels before initiating treatment Arm B.
A single dose PK run-in will be required for subjects enrolled into the first 3 cohorts of each treatment arm. Subjects will be treated at increasing doses of study drug until all dose levels have been investigated or any dose level is found to exceed the maximum tolerated dose (MTD). A RP2D will be selected from one of the tolerable dose levels which will not exceed the MTD (see Figure 1 for study design).
Key study inclusion criteria include: Age ≥ 18 years of age; Life expectancy ≥ 8 weeks; Confirmed diagnosis of R/R AML or HR and VHR MDS; Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2. Key study exclusion criteria include: Active malignancies not related to AML or MDS within two years prior to the first dose or requiring ongoing treatment; Clinical evidence of central nervous system (CNS) or pulmonary leukostasis; ≥ Grade 3 disseminated intravascular coagulation, or active CNS leukemia; QT interval corrected for heart rate per Fridericia's formula ≥470 msec during screening ECG.
The study primary objectives are to evaluate the safety and tolerability of SGR-2921 as monotherapy and identify RP2D including MTD. Secondary objectives include evaluating the pharmacokinetics (PK) of SGR-2921 and investigating preliminary antitumor activity (composite complete remission rate, objective response rate, duration of response, etc.).
Disclosures
DiNardo:ImmuniOnc: Honoraria; AbbVie/Genentech: Honoraria; Notable Labs: Honoraria; Astellas: Honoraria; Takeda: Honoraria; BMS: Honoraria; Schrödinger: Consultancy; Fogham: Honoraria; Servier: Honoraria; Novartis: Honoraria. Strickland:Abbvie: Consultancy; BerGen Bio: Consultancy; Genentech: Consultancy; Novartis: Consultancy; SentiBio: Consultancy; NKarta: Other: Mentoring. Skikne:Schrödinger: Consultancy. Zeidan:Orum: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Astex: Research Funding; Incyte: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Shattuck Labs: Research Funding; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Syros: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Notable: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Foran: Consultancy, Research Funding. Traer:Incyte: Research Funding; Astra-Zeneca: Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Schrodinger: Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Prelude Therapeutics: Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Carraway:Novartis: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau; Genentech: Consultancy; Astex Pharmaceuticals: Other; AbbVie: Other; Agios: Consultancy, Speakers Bureau; Syndax: Other: DSMB; Stemline Therapeutics: Consultancy, Speakers Bureau; Celgene: Research Funding; BMS: Consultancy, Research Funding, Speakers Bureau; Daiichi: Consultancy; Takeda: Other. Frankel:Schrödinger: Consultancy. Weiss:Schrödinger: Current Employment. Wang:Schrödinger: Current Employment. Pirie-Shepherd:Schrödinger: Ended employment in the past 24 months. Piccotti:Schrödinger: Current Employment. Wright:Schrödinger: Current Employment. Akinsanya:Schrödinger: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal